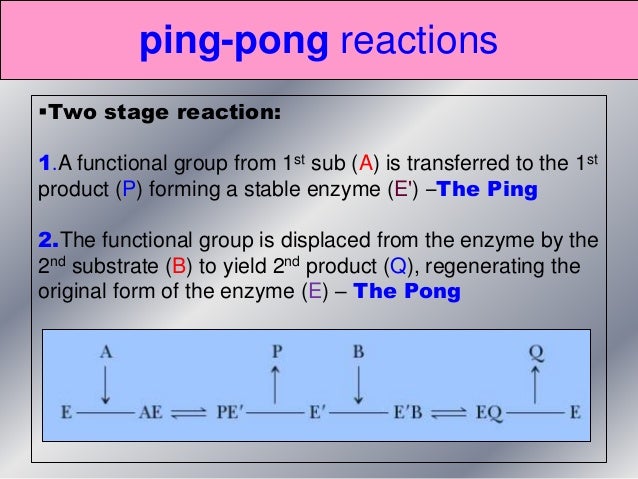
Ping Pong Mechanism Enzyme Kinetics

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen

What Is A Bisubstrate Enzyme Reaction And Double Displacement Reaction Quora

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen

Enzyme Kinetics Wikipedia

Structural Biochemistry Enzyme Regulation Wikibooks Open Books For An Open World

Kinetic Mechanisms Enzyme Molecular Biology
The first was the catalytic promiscuity of substrates (Pandya et al.

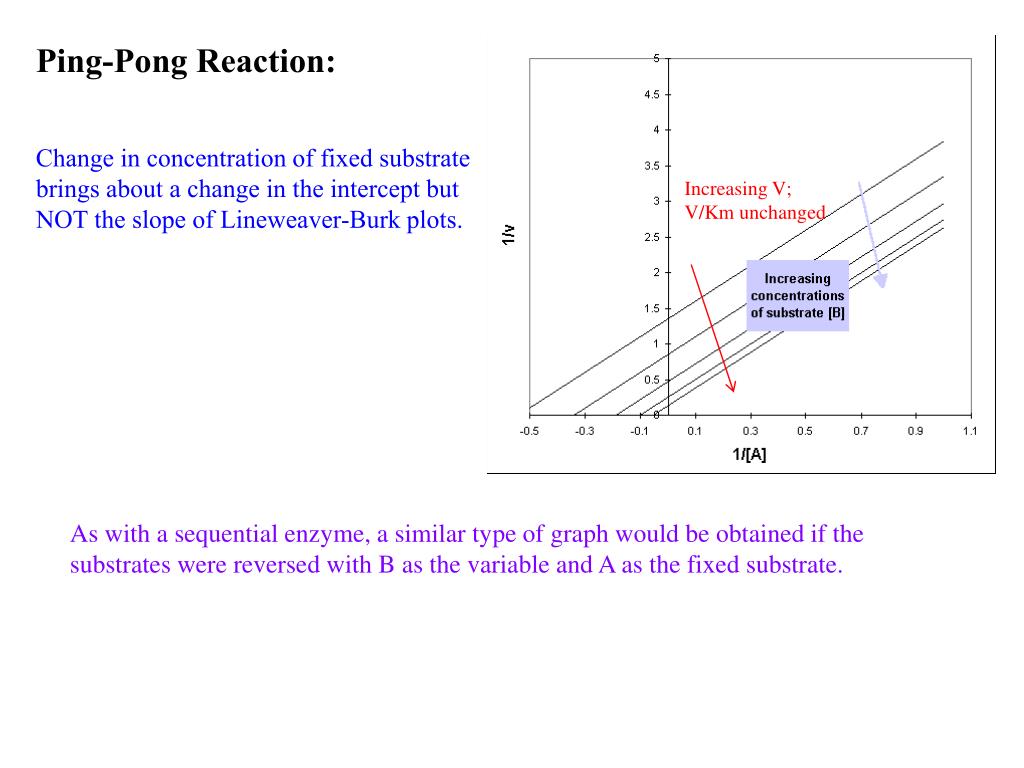
Ping pong mechanism enzyme kinetics. Ping-pong mechanism of bisubstrate enzymatic reactions. He pioneered the utilization of heavy atom isotope effects for the elucidation of. Other mechanisms can commonly give mixed inhibition.
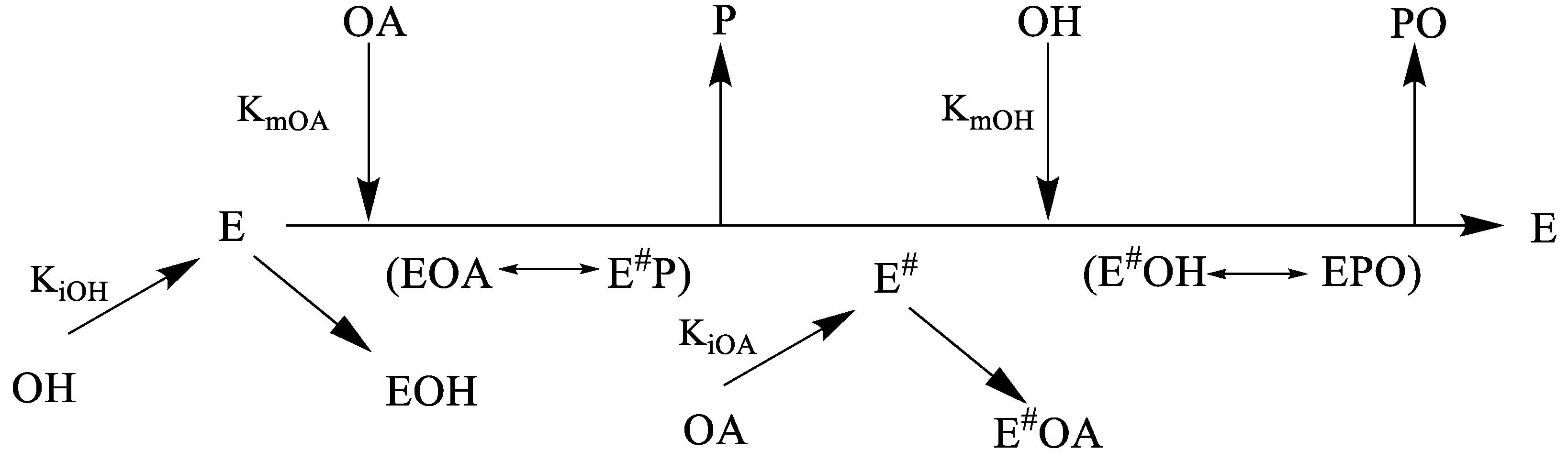
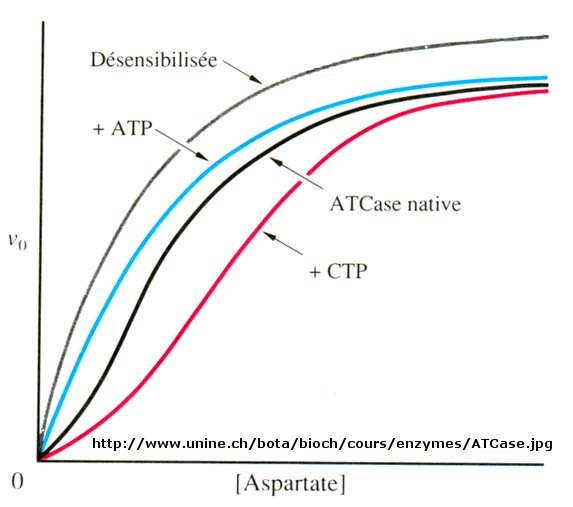
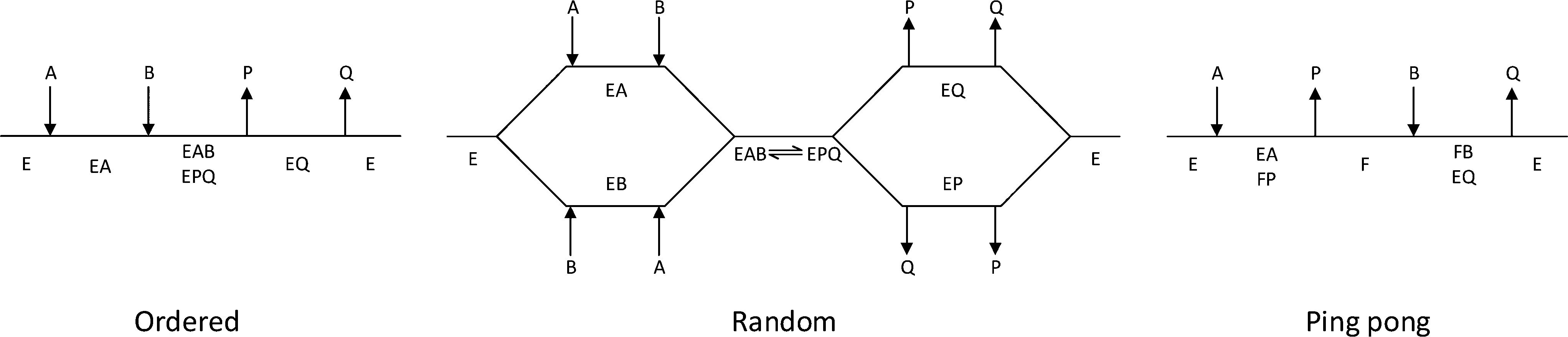
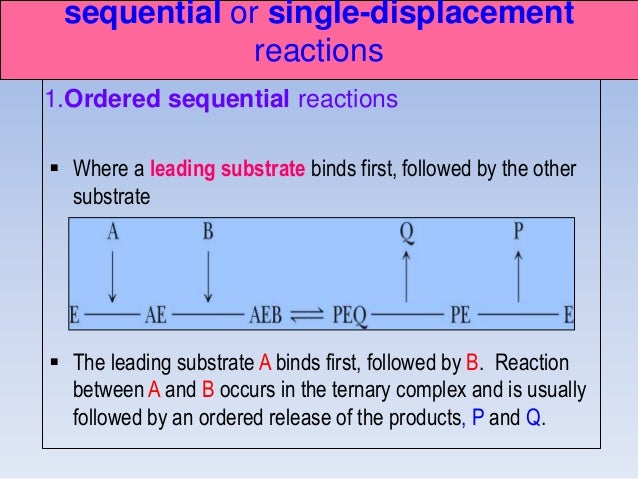
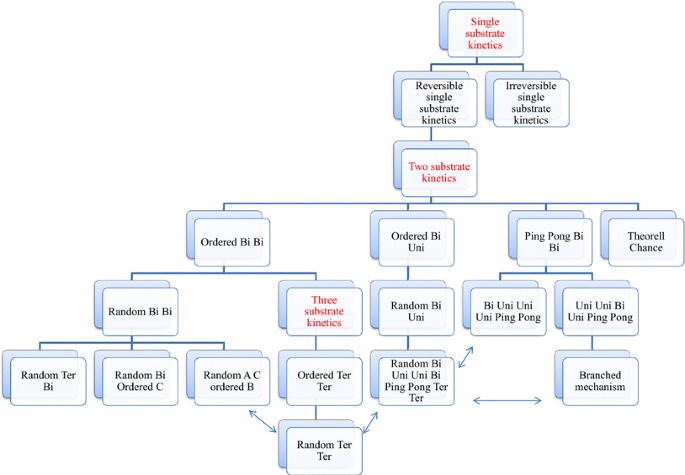
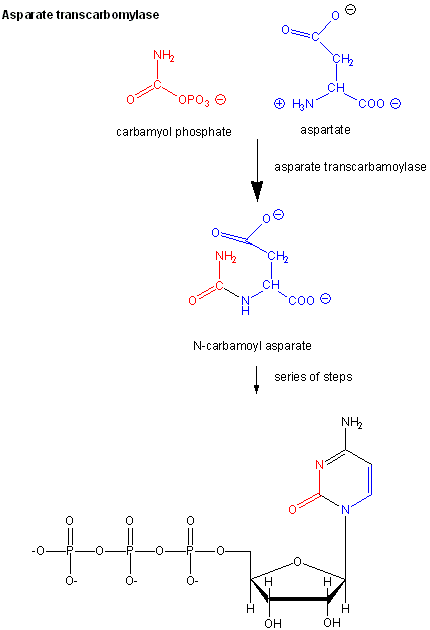
The ordered mechanisms are those in which the reaction substrates bind to the enzyme in a defined order. For instance, for a bi-substrate enzyme, it is possible to realize whether both substrates must bind to the enzyme before giving any products (ternary-complex mechanism) or not (ping-pong mechanism). If P, acting as a product inhibitor, can bind to two different forms of the enzyme (E' and also E), it will act as an mixed inhibitor.
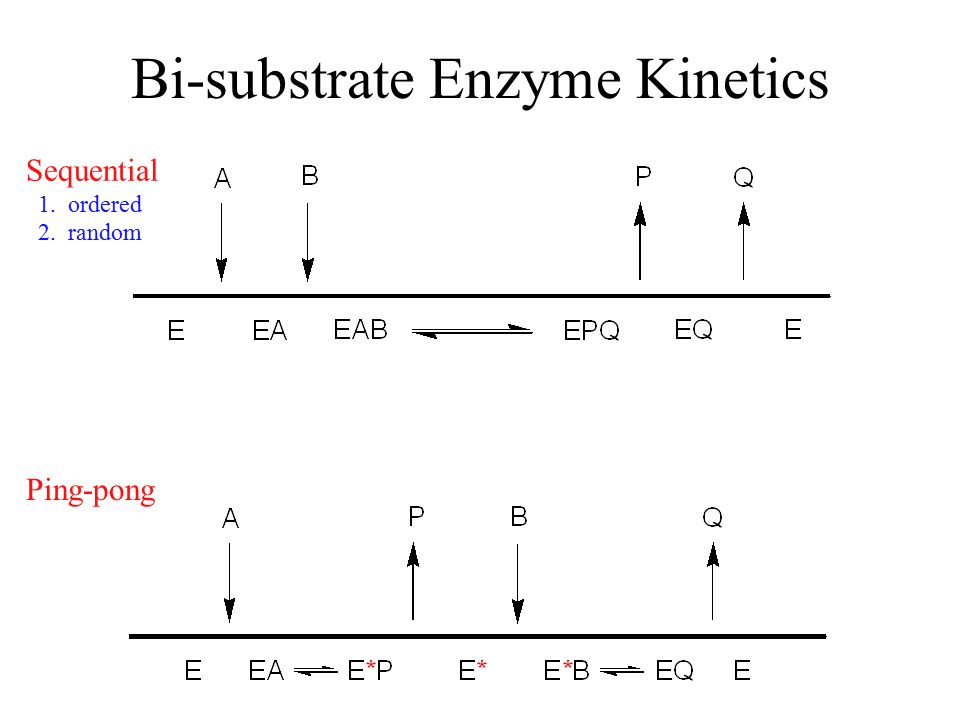
Kinetics of Bi-substrate reaction, Ping-Pong reaction, multi-substrate reaction. Sequential kinetic mechanisms for human Icmt. The following equation can be derived from ping-pong bi-bi mechanism.
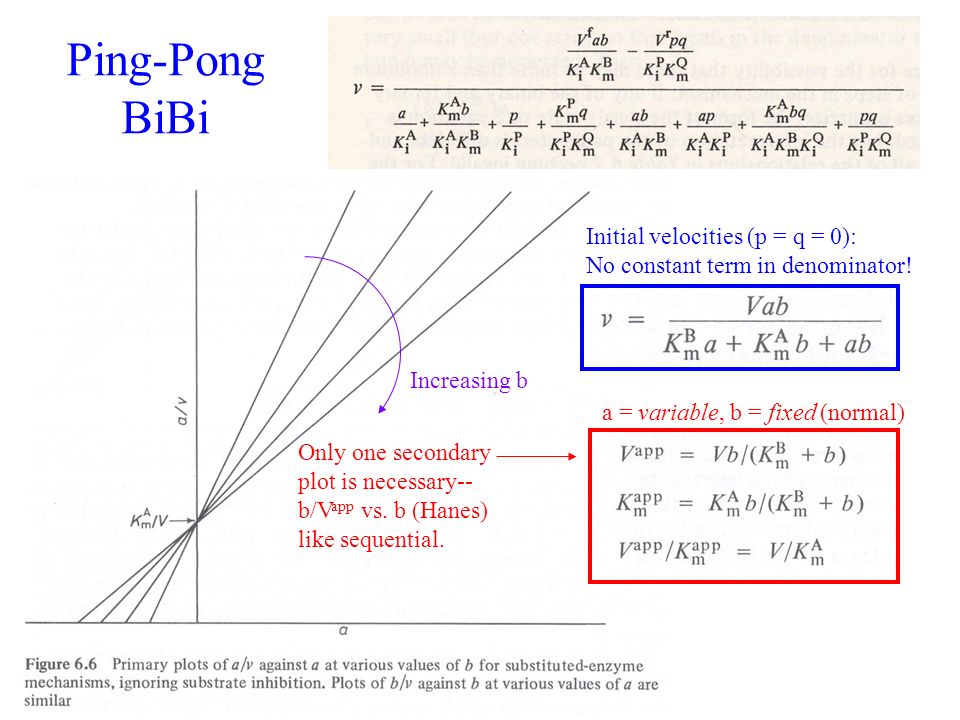
He will be most remembered for giving the enzyme community Ping-Pong kinetics and the invention of dithiothreitol (DTT). The ping-pong mechanism is a non-sequential mechanism. \ v =\dfrac{V_M AB}{K_bA + K_aB + AB}\ For simplicity, all of the enzyme kinetic equations have been derived assuming no products are present.
One property of both the random-ordered and the compulsory-ordered reaction is that a ternary complex involving the two substrates and the enzyme form prior to the release of any products. Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes, with a focus on their reaction rates.The study of an enzyme's kinetics reveals the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a poison might inhibit the enzyme. Enzymes are protein molecules that manipulate other.
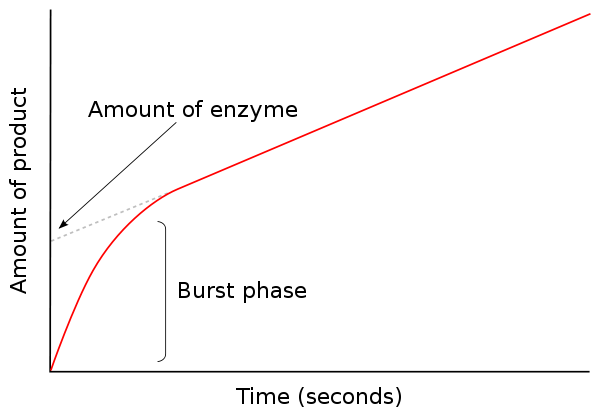
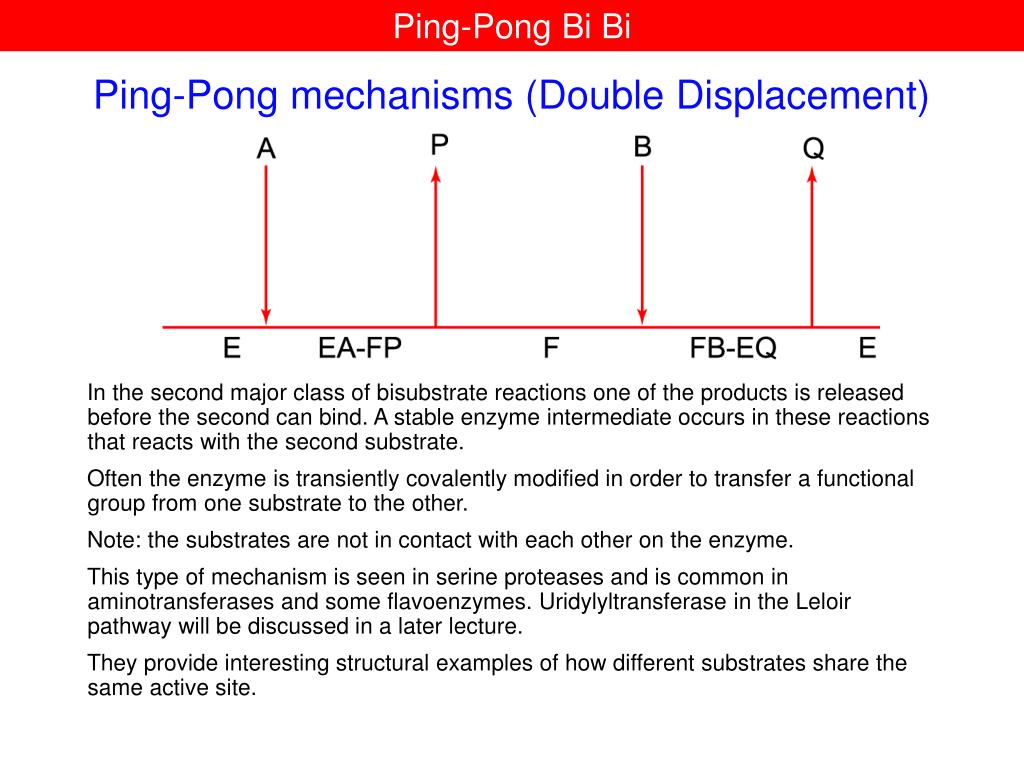
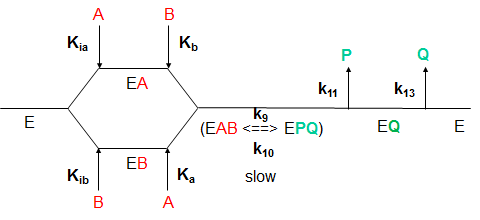
• Catalysis is the process of accelerating a reaction by lowering the energy of activation (E a ). For example, a ping–pong mechanism with burst-phase pre-steady-state kinetics would suggest covalent catalysis might be important in this enzyme's mechanism. KlA k3 k6 E'k2EAZ.FP-F ksp SCHEME I where E = enzyme, A = substrate, P = product, and F is the stable enzyme form which contains the portion of A which will eventually be transferred to the second substrate.
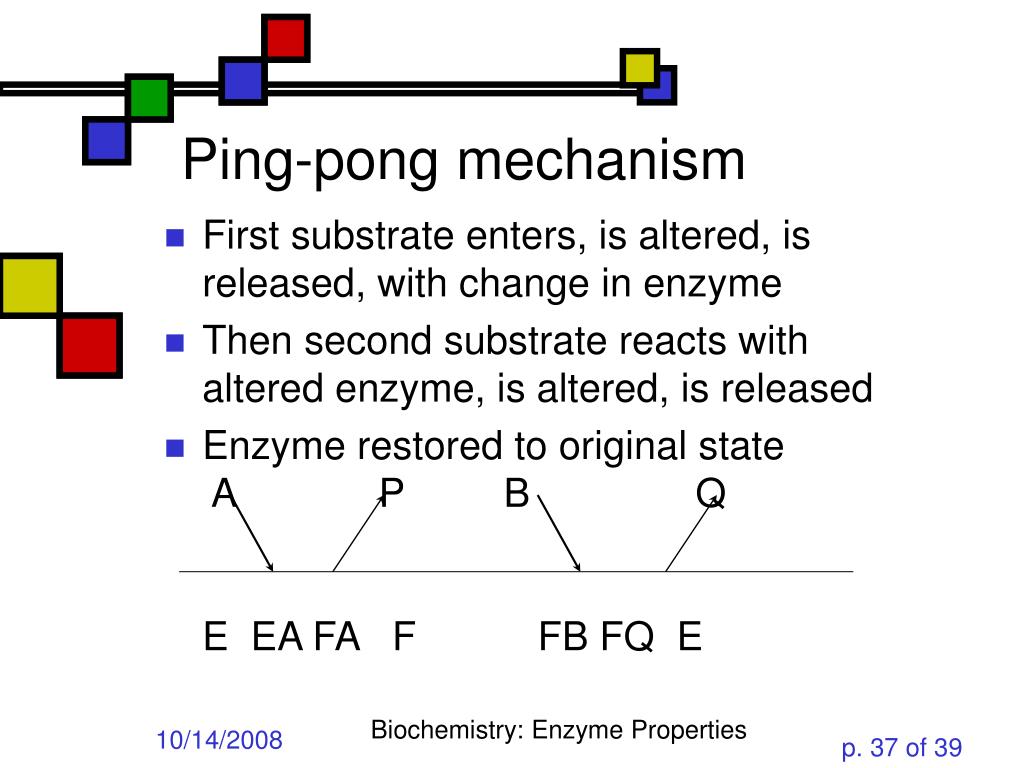
The patterns obtained give insights into the kinetic mechanism involved. A product is released after the first substrate is bound. In reality, many enzymes have more than one substrate (A, B) and more than one product (P, Q).For example, the enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol with NAD (a biological oxidizing agent) to form acetaldehyde and NADH.
You will be given the Michaelis Menten equations on the exam formula sheet.) 1. Enzymes (Part 2 of 5) - Enzyme Kinetics and The Michaelis Menten Model. The rest of the substrate is covalently attached to the enzyme E, which is designated as E'.
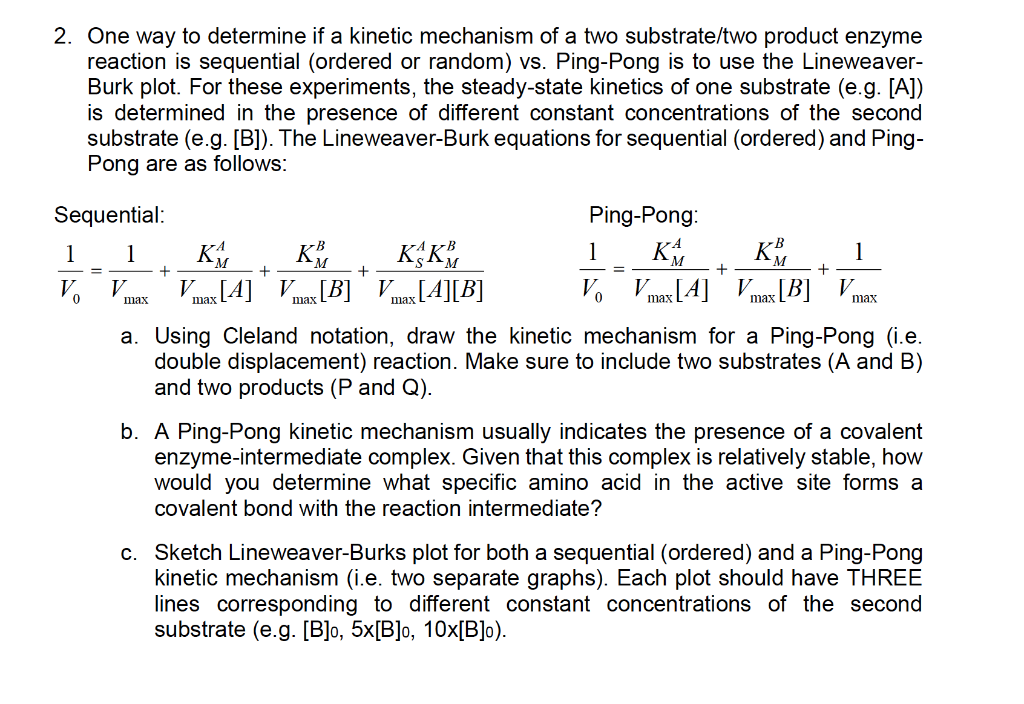
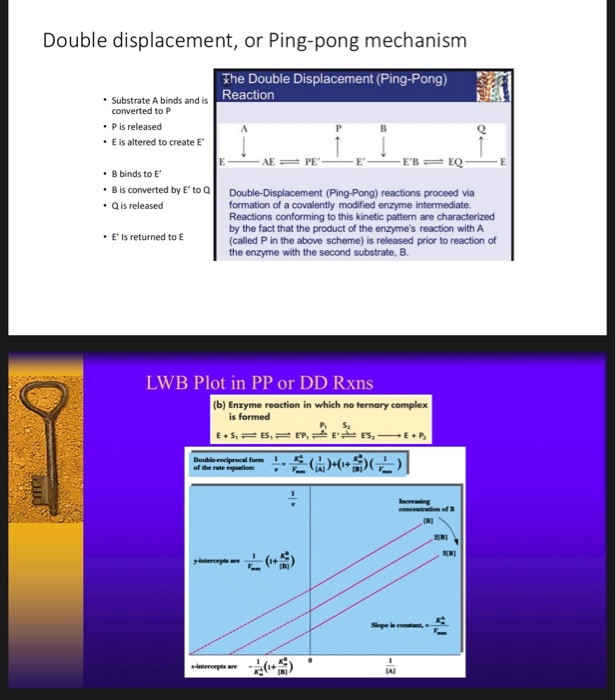
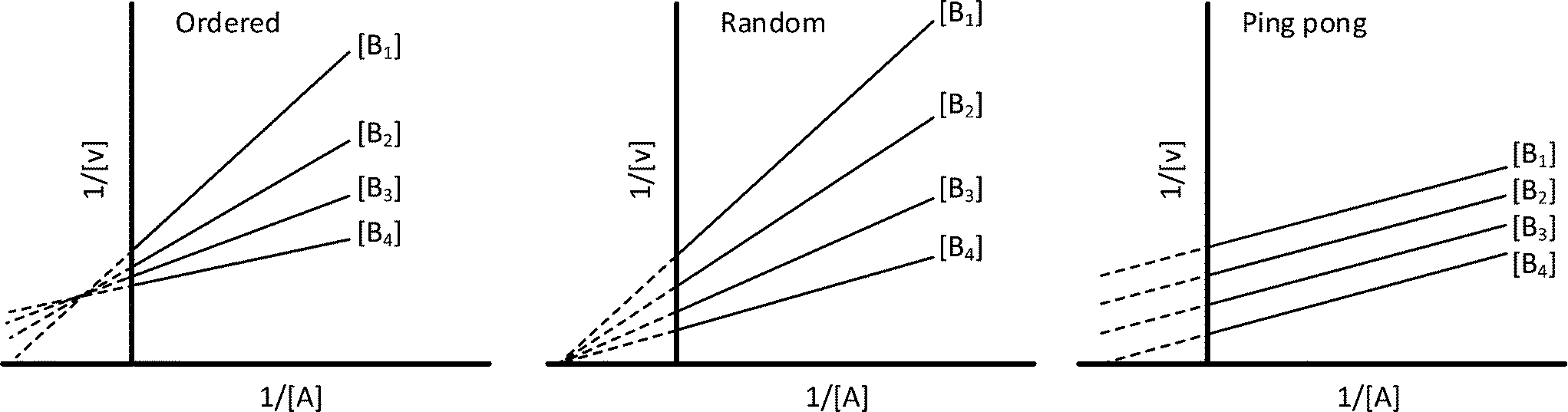
Look at other dictionaries:. Crossover Point Analysis Terreactant Enzyme Mechanisms No Constant Term-Ping Pong Mechanisms Constant Term Present-Sequential Mechanisms Methods for Telling Which Denominator Terms Are Missing Haldane Relationship Alternate Substrate Studies Kinetics of Metal Ions Cooperativity and Allosterism Positive Cooperativity Negative Cooperativity The. Lineweaver-Burk plot for the methylation of BFC carried out in the presence of ( ).
Enzymes with a ping-pong mechanism can exist in two states, E and a chemically modified form of the enzyme E*;. Enzyme kinetics cannot prove which modes of catalysis are used by an enzyme. GR may change from using a sequential mechanism to a ping-pong mechanism (Rakauskiene 19) or a hybrid ping-pong semi-random mechanism (Ozer and Ogus 01).
Two, binding of the first substrate causes the enzyme to change into an intermediate form that will bind the second substrate. FIGURE 6-14b Steady-state kinetic analysis of bisubstrate rxns. The enzyme produces product at an initial rate that is approximately linear for a short period after the start of the reaction.
For example, a ping–pong mechanism with burst-phase pre-steady-state kinetics would suggest covalent catalysis might be important in this enzyme's mechanism. Typically, product P is a fragment of the original substrate A. This modified enzyme is known as an intermediate.
• Enzyme kinetics refers to the catalytic behavior of enzymes, specifically focusing on reaction rates. However, some kinetic data can suggest possibilities to be examined by other techniques. (redirected from Ping-pong reaction) en·zyme ki·net·ics the study of the rates, and alterations in those rates, of enzyme-catalyzed reactions;.
Includes the reactions catalyzed by synzymes, abzymes, and ribozymes. Enzymes are usually protein molecules that manipulate other molecules — the. Ping pong Mechanism.
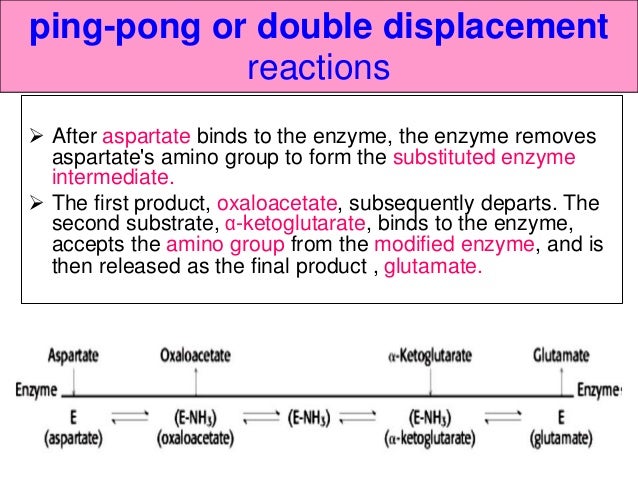
A ping-pong mechanism integrates phosphorylation of a histidine residue by transferring terminal phosphate group (γ-phosphate) from ATP to NDP β-phosphate in order to produce a NTP, and NDPK catalyzes such reversible reactions. Ping-Pong Mechanism In this mechanism, one substrate binds first to the enzyme followed by product P release. Or does the transfer occur in a single step while both donor and acceptor are still in the active site of the enzyme?.
It might be simpler to calculate an IC50 and determine apparent Km. Enzyme Kinetics, Athel Cornish-Bowden and C. Although a single substrate is involved, the existence of a modified enzyme intermediate means that the mechanism of catalase is actually a ping–pong mechanism, a type of mechanism that is discussed in the Multi-substrate reactions section below.
In this work we have examined only ordered ping-pong mechanism. If the enzyme is saturated with substrate, the highest k2 is most efficient - in living cells S < Km, so rate is << k2 - if high k2/Km ratio, catalysis occurs at rate equal to highest diffusion rate of E and S, 10^9 (M^-1 s^-1) High k2/Km have reached kinetic perfection Specificity Constant:. This is repeated for several values of S2, generating several separate lines.
This mechanism is supported by affinity measurements of both substrates using isothermal titration calorimetry (ITC). Enzyme Mechanism Ping-pong bi bi E E A (ester) P (alcohol) B (water) Q (acid) F (E–Q = F) Acylation Deacylation What is the nature of this enzyme intermediate (F)?. The cardinal feature of this mechanism is that the product (P) dissociates from the enzyme before the addition of the second substrate.Double reciprocal plots, of the type illustrated in Fig.
In this second case, is the order of binding of donor and acceptor. The evolution of the kinetic mechanisms of enzymes included two important steps. This is in contrast to the ping-pong, or enzyme displacement, mechanism where one product is released prior to the binding of the second substrate.
Enzymatic reactions requiring multiple substrates and yielding multiple products are more common and yielding multiple products are more common than single-substrate reaction. In the Bi Bi Ping-Pong mechanism, shown in Fig. However, some kinetic data can suggest possibilities to be examined by other techniques.
For example, a ping–pong mechanism with burst-phase pre-steady-state kinetics would suggest covalent catalysis might be important in this enzyme's mechanism. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might. One, a product is seen before the second substrate is bound.
Ping Pong Bi Bi Mechanism • Intercept changes because A and B bind to the different enzyme forms E and F, respectively • Slope remains same because the binding of A and B is irreversible due to the release of the product (P). The kinetic experiments indicate a ping-pong mechanism in which the enzyme binds Ac-CoA first, followed by binding of the histone substrate. Enzyme kinetics cannot prove which modes of catalysis are used by an enzyme.
Ping-pong or sequential mechanisms of GR or those of other enzymes that act similar to GR are referred to as branched kinetic mechanism (Mannervik 1973). Enzymes with ping–pong mechanisms include some oxidoreductases such as thioredoxin peroxidase, transferases such as acylneuraminate cytidylyltransferase, and serine proteases such as trypsin and chymotrypsin. Bio40 Problem Set 3 Problems on Enzyme kinetics and mechanism (note:.
Computational systems biology 5. Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes, with a focus on their reaction rates.The study of an enzyme's kinetics provides insights into the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled in the cell, and how drugs and poisons can inhibit its activity. However, some kinetic data can suggest possibilities to be examined by other techniques.
Enzyme kinetics cannot prove which modes of catalysis are used by an enzyme. Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes.In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. In such mechanisms, substrate A binds, changes the enzyme to E* by, for example, transferring a chemical group to the active site, and is then released.
In the case of pyranose-2-oxidase, in step 1 glucose binds to the enzyme containing. (Book chap 12#9) At what. 5.27, yield a family of parallel lines.
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes, with a focus on their reaction rates.The study of an enzyme's kinetics provides insights into the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled in the cell, and how drugs and poisons can inhibit its activity. Enzyme kinetics studies the speed of the reactions catalyzed by enzymes. (redirected from Ping-pong reaction mechanism) en·zyme ki·net·ics the study of the rates, and alterations in those rates, of enzyme-catalyzed reactions;.
V = (Vmax A B)/ (KmA B+ KmB A+ A B). State enzyme kinetics, died on March 6, 13, from injuries sustained in a fall outside of his home. Enzyme kinetics is the study of the rates of chemical reactions that are catalysed by enzymes.The study of an enzyme's kinetics provides insights into the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled in the cell and how drugs and poisons can inhibit its activity.
And also remember that for this to happen the reacting substrate, which I called S, will bind to the enzyme E to form the. What fraction of V MAX is observed at S = 0.5 K M, S = 1 K M, S = 2 K M, S = 4 K M?. When products are absent, if Kmtends to zero as the concentration of the changing fixed substrate tends to zero, then the mechanism is ping pong.
For instance, for a bi-substrate enzyme, it is possible to realize whether both substrates must bind to the enzyme before giving any products (ternary-complex mechanism) or not (ping-pong mechanism). NDPK utilize specific enzyme kinetics for multi-substrate reaction, namely ping-pong mechanism. Michaelis Menten equations are given in the powerpoint presentation on Enzyme kinetics, as well as in the textbook.
For example, the product released in a ping pong mechanism (discussed in the next chapter) can give mixed inhibition. You need to know if the binding of substrates is ordered or random and if the enzyme utilizes a ping-pong mechanism. As the reaction proceeds and substrate is consumed, the rate continuously slows (so long as substrate is not still at saturating levels).
In these double-reciprocal plots (see Box 6-1), the concentration of substrate 1 is varied while the concentration of substrate 2 is held constant. For more Biochemistry videos:. Kcat/Km - limited by diffusion from active site.
The Michaelis –Menten model of enzyme kinetics was derived for single substrate reactions. So we're going to talk about enzyme kinetics today, but first let's review the idea that enzymes speed up reactions by lowering the delta G of the transition state, or lowering the activation energy of a reaction. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might.
Ping-pong or sequential mechanisms of GR or those of other enzymes that act similar to GR are referred to as branched kinetic mechanism (Mannervik 1973). Enzymes are protein molecules that manipulate other. Wharton, IRL Press, 19.
The velocity equation for a 2-substrate Ping Pong reaction is:. 5.26, substrate A reacts with the enzyme to form an intermediate that may or may not be covalent. Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes.In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated.
The ping-pong mechanism can be categorized into two groups, namely random ping- pong and ordered ping-pong mechanisms. Includes the reactions catalyzed by synzymes, abzymes, and ribozymes. • Kinetic mechanism This is called pre-steady state kinetics Chymotrypsin • React with acylating reagent Enzyme Mechanism• Protein modification studies • Denature the.
Determination of Kinetic Mechanism The mechanism of a multi-substrate enzyme is designated as either ping-pong if the reaction proceeds with the release of one or more products prior to the association of all substrates, or as ternary complex (or sequential) if all substrates bind to the enzyme prior to the formation of the first product. Ternary complex (B) 1. Mechanism of Chymotrypsin - Duration:.
Enzyme Kinetics Wikiwand
Http Personal Tcu Edu Yryu Kinetics Pdf
Http Personal Tcu Edu Yryu Kinetics Pdf

Research
Fermentation Free Full Text Optimization Of The Enzymatic Synthesis Of Pentyl Oleate With Lipase Immobilized Onto Novel Structured Support

Kinetic Mechanisms Enzyme Molecular Biology

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen
Q Tbn 3aand9gcrq0b9pzpxqvxgh2 Vmkai Oth7ektavfyo Em7il7zyf33bntr Usqp Cau
Ppt Enzyme Properties And Kinetics Powerpoint Presentation Free Download Id
2 One Way To Determine If A Kinetic Mechanism Of Chegg Com

Schematic Representation Of The Ping Pong Bi Bi Mechanism With Download Scientific Diagram
Add Your Page Title
Www Jbc Org Content Early 17 11 30 Jbc M117 Full Pdf

Kinetic Analysis Kinetics Of Multisubstrate Reaction Theorell Chance Sequential Mechanics

Enzyme Mechanism An Overview Sciencedirect Topics

Enzyme Kinetics And Inhibition Of Histone Acetyltransferase Kat8 Sciencedirect

Kinetics Of Spanish Broom Peroxidase Obeys A Ping Pong Bi Bi Mechanism With Competitive Inhibition By Substrates Sciencedirect
Www Inf Ed Ac Uk Teaching Courses Csb Csb Lecture Enzyme Kinetics Pdf

Enzyme Kinetics Wikipedia

Enzyme Kinetics And Inhibition Of Histone Acetyltransferase Kat8 Sciencedirect
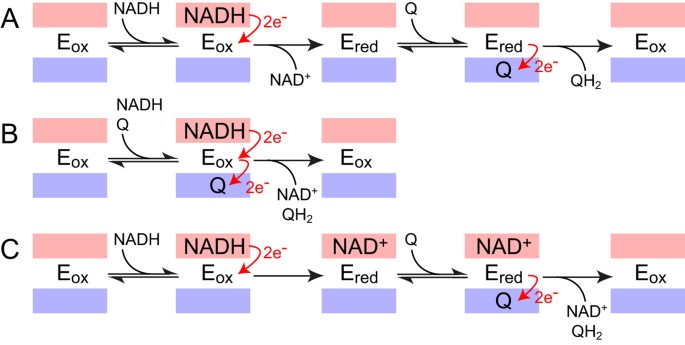
The Mechanism Of Catalysis By Type Ii Nadh Quinone Oxidoreductases Scientific Reports
Q Tbn 3aand9gctqomucmtmqb62j006 2li8cpwmtiiqzxfk2vqel7s99ypvq3gf Usqp Cau

Figure 3 From The Incomplete Glutathione Puzzle Just Guessing At Numbers And Figures Semantic Scholar
Bisubstrate Reactions Enzyme Kinetics

Multisubstrate Reactions Notsaem

Module Quiz 9 Steady State Kinetics Flashcards Quizlet
Q Tbn 3aand9gcswpc5rbrx 5zgil Uh0d2vok4i Gme Ijuhrussduca4xjcqqb Usqp Cau

Enzyme Kinetics Wikipedia

Galangin Competitively Inhibits Xanthine Oxidase By A Ping Pong Mechanism Semantic Scholar
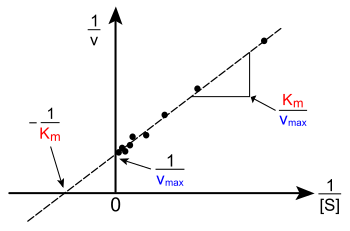
Enzyme Kinetics Wikipedia

Multi Substrate Enzyme Kinetics Ppt Video Online Download

Control Theory Concepts For Modeling Uncertainty In Enzyme Kinetics Of Biochemical Networks Biorxiv
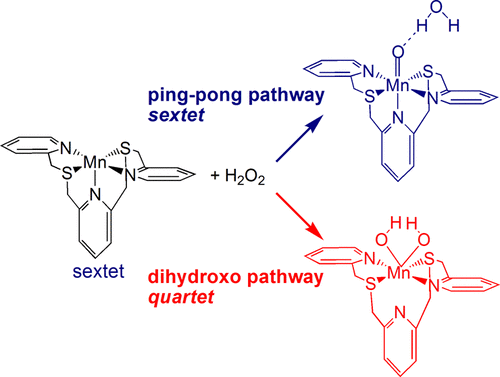
Understanding The Catalase Like Activity Of A Bioinspired Manganese Ii Complex With A Pentadentate Nsnsn Ligand Framework A Computational Insight Into The Mechanism Acs Catal X Mol

New Types Of Experimental Data Shape The Use Of Enzyme Kinetics For Dynamic Network Modeling Tummler 14 The Febs Journal Wiley Online Library
The Application Of Reaction Engineering To Biocatalysis Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 C5rea
Www Beilstein Institut De Download 691 Rohwer 1 Pdf

Distinguishing Ping Pong Vs Sequential Kinetic Mechanisms For Human Download Scientific Diagram
Bisubstrate Reactions Enzyme Kinetics

Ping Pong Catalytic Mechanism For Kasa Acyl Enzyme Formation Occurs Download Scientific Diagram
Spire And Formin 2 Synergize And Antagonize In Regulating Actin Assembly In Meiosis By A Ping Pong Mechanism

Evolution Of Enzyme Kinetic Mechanisms Abstract Europe Pmc
Bisubstrate Reactions Enzyme Kinetics

Enzyme Kinetics Wikiwand
Q Tbn 3aand9gcr39kxaj6apmey3vzsr8rmbwxslrm3qt9ttslew Xsnw6ftkyud Usqp Cau
Ppt Bisubstrate Enzymes Powerpoint Presentation Free Download Id 472
D More Complicated Enzymes
Sensors Free Full Text Mediated Electron Transfer At Redox Active Monolayers Part 4 Kinetics Of Redox Enzymes Coupled With Electron Mediators Html

Two Possible Bi Ter Ping Pong Uni Uni Uni Bi Kinetic Mechanisms For Dna Download Scientific Diagram
Evolution Of Enzyme Kinetic Mechanisms Springerlink

Control Theory Concepts For Modeling Uncertainty In Enzyme Kinetics Of Biochemical Networks Biorxiv
Solved Amino Transferases Are Considered A Double Displac Chegg Com
The Application Of Reaction Engineering To Biocatalysis Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 C5rea
Http People Uleth Ca Steven Mosimann hm3100 hm3100 L8 Pdf

Multi Substrate Enzyme Kinetics Ppt Video Online Download
Deriving Kinetic Parameters Rate Equations For Multi Substrate Systems Ppt Download

Substrate Inhibition Molecular Biology
Add Your Page Title
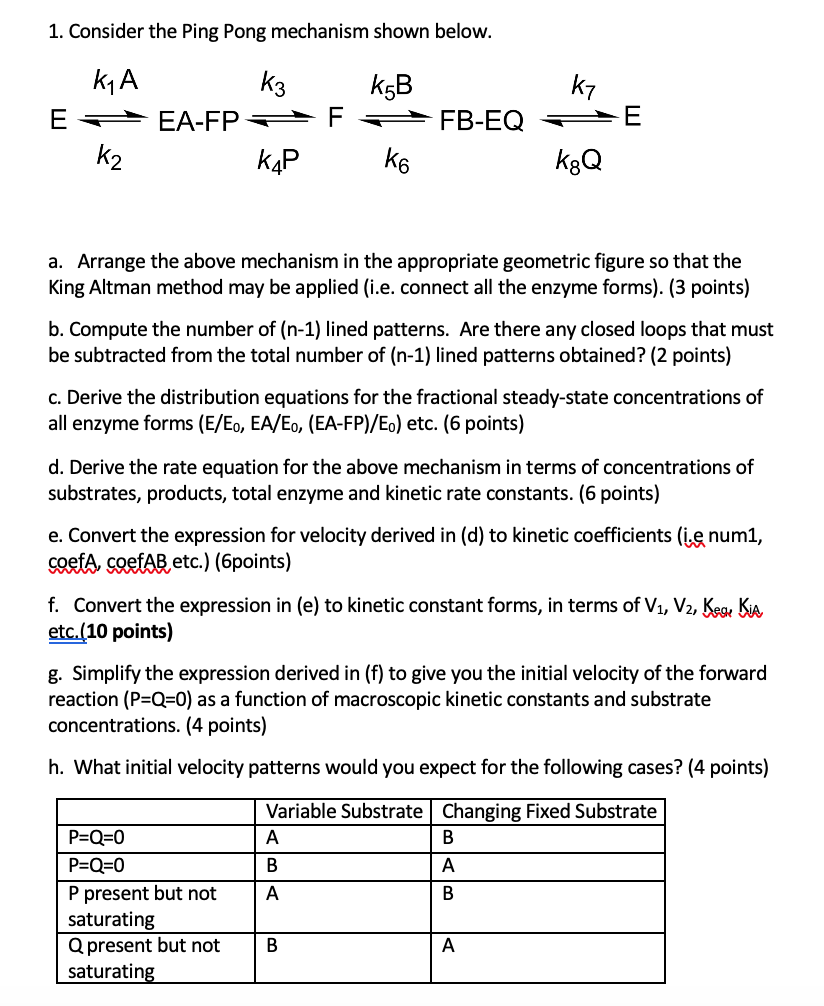
1 Consider The Ping Pong Mechanism Shown Below K1 Chegg Com
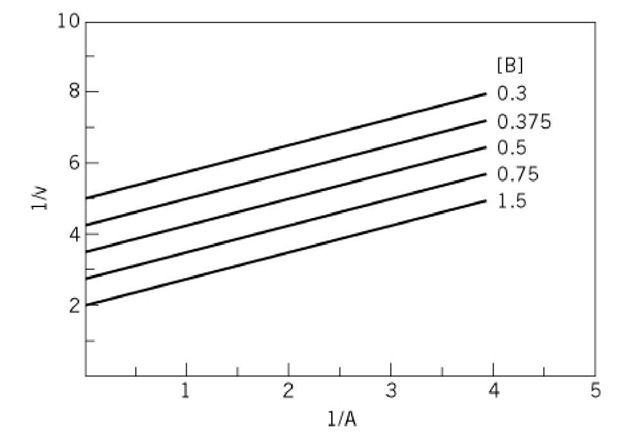
Kinetic Mechanisms Enzyme Molecular Biology

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen
Add Your Page Title

Derivation And Identification Of A Mechanistic Model For A Branched Enzyme Catalyzed Carboligation Ohs 19 Biotechnology Progress Wiley Online Library

Analysis Of A Dual Domain Phosphoglycosyl Transferase Reveals A Ping Pong Mechanism With A Covalent Enzyme Intermediate Pnas
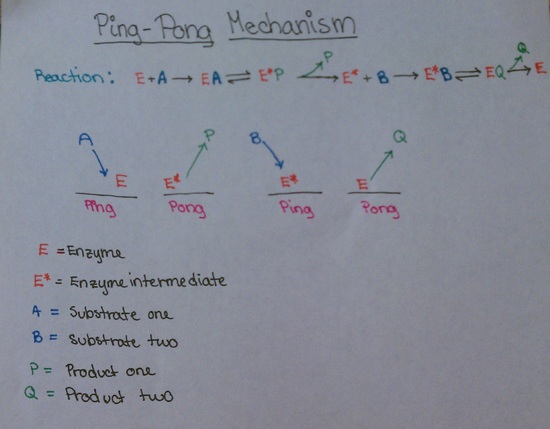
The Ping Pong Mechanism Chemistry Libretexts
Link Springer Com Content Pdf 10 1007 2f0 306 490 4 16 Pdf

350kineticsnotes

Structural Biochemistry Enzyme Catalytic Mechanism Pingpong Mechanism Wikibooks Open Books For An Open World
Http Personal Tcu Edu Yryu Kinetics Pdf

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen
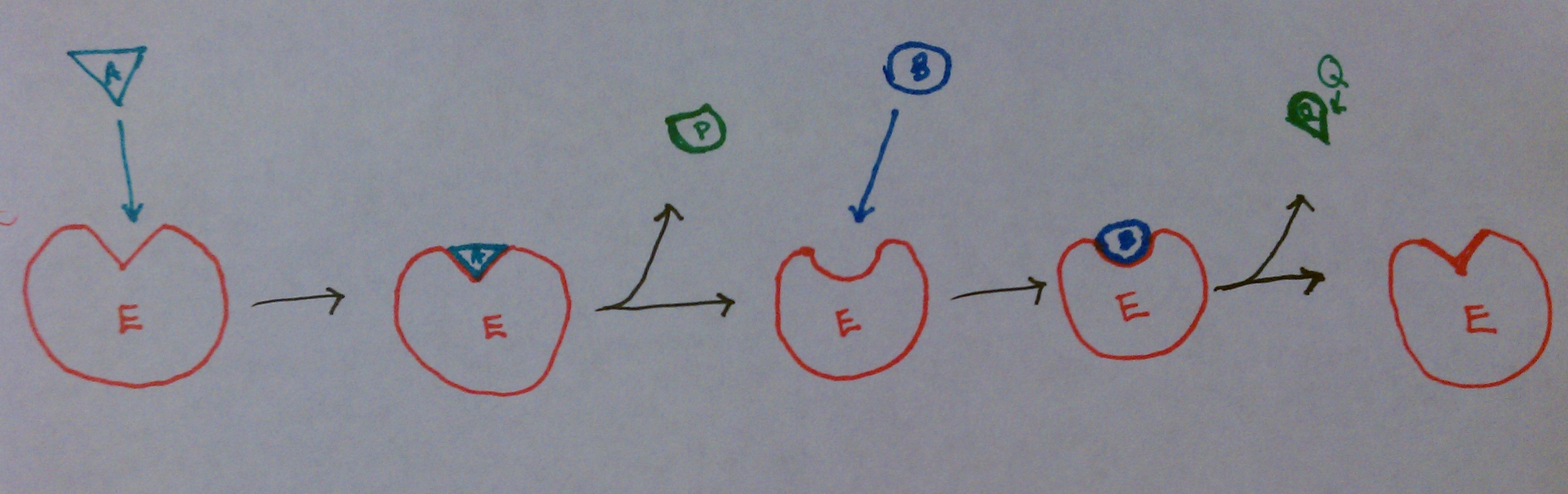
The Ping Pong Mechanism Chemistry Libretexts
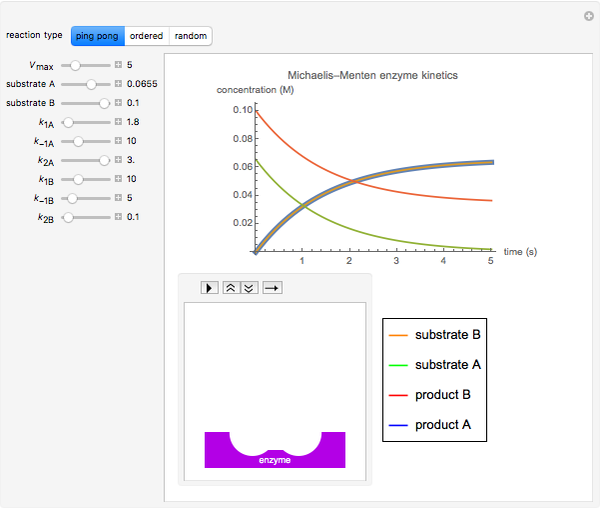
Michaelis Menten Enzyme Kinetics Of Bi Bi Reactions Wolfram Demonstrations Project

7 29 10 Enzymes Kinetics Coloso
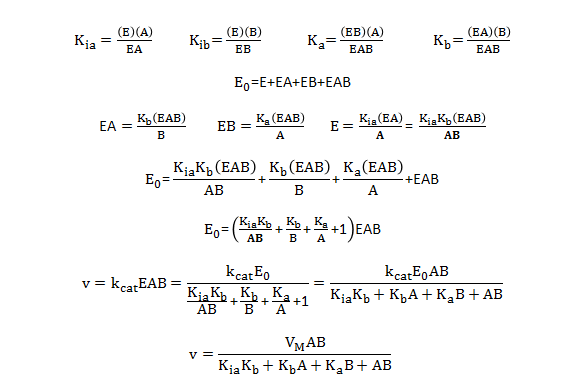
Add Your Page Title
Kinetic Study Of Lipase Catalyzed Glycerolysis Of Palm Olein Using Lipozyme Tlim In Solvent Free System
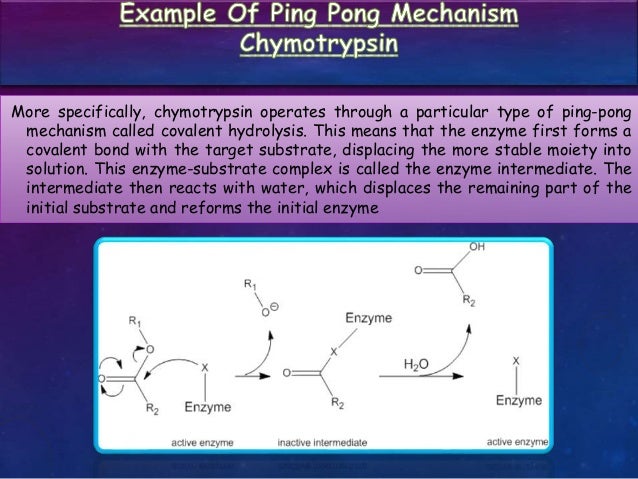
Kinetics Of Multi Substrate Enzyme Catalyzed Reaction
Http Personal Tcu Edu Yryu Kinetics Pdf
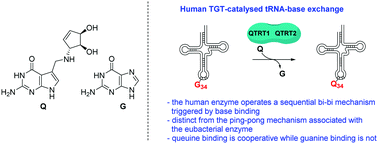
The Eukaryotic Trna Guanine Transglycosylase Enzyme Inserts Queuine Into Trna Via A Sequential Bi Bi Mechanism Chemical Communications Rsc Publishing
Ppt Multi Substrate Enzyme Kinetics Powerpoint Presentation Free Download Id
Kinetic Study Of Lipase Catalyzed Glycerolysis Of Palm Olein Using Lipozyme Tlim In Solvent Free System
Bi Substrate Enzyme Kinetics Ppt Video Online Download

Lnt Follows A Ping Pong Mechanism Double Reciprocal Plot Showing Download Scientific Diagram
Http People Uleth Ca Steven Mosimann hm3100 hm3100 L8 Pdf

Analysis Of A Dual Domain Phosphoglycosyl Transferase Reveals A Ping Pong Mechanism With A Covalent Enzyme Intermediate Pnas

Ping Pong Mechanism Half Reactions Of Oat E Plp Oat Plp Adduct Download Scientific Diagram
Add Your Page Title

Kinetics Of Spanish Broom Peroxidase Obeys A Ping Pong Bi Bi Mechanism With Competitive Inhibition By Substrates Sciencedirect

Enzyme Kinetics Biochem Exam 2 Flashcards Quizlet

Here

Enzyme Kinetics Wikipedia

Team Scut China Modeling Simulation 14 Igem Org
Http Www Ijcce Ac Ir Article 57 f04d9ffa518ff66d63af0f Pdf

350kineticsnotes
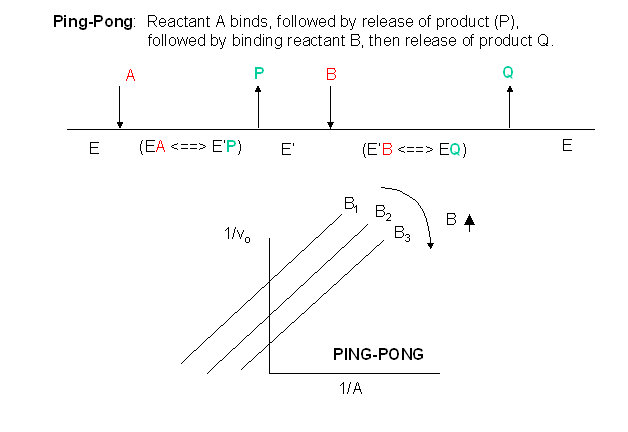
D2 Multi Substrate Ping Pong Mechanisms Biology Libretexts

Kinetic Analysis Kinetics Of Multisubstrate Reaction Theorell Chance Sequential Mechanics
Http Lascu Free Fr Enzymology 02b basic equations two substrates Pdf

Kinetic Modelling Of Enzyme Catalyzed Biotransformation Involving Activations And Inhibitions Intechopen

Multisubstrate Reactions Notsaem

Two Representations Of A Ping Pong Bi Bi Enzymatic Reaction A Network Download Scientific Diagram



